We define a foldamer as any non-natural compound that can fold into a well-defined three-dimensional structure. Folding may be driven by a combination of attractive and/or repulsive intramolecular interactions, solvophobic effects, binding to another ion/molecule, or an externally applied stimulus such as light or an electric field.
This is a broad definition that not only includes peptidic and oligonucleotide systems, as well as abiotic foldamers, but can also cover architectures such as aptamers, molecularly imprinted polymers, and molecular machines and many others.
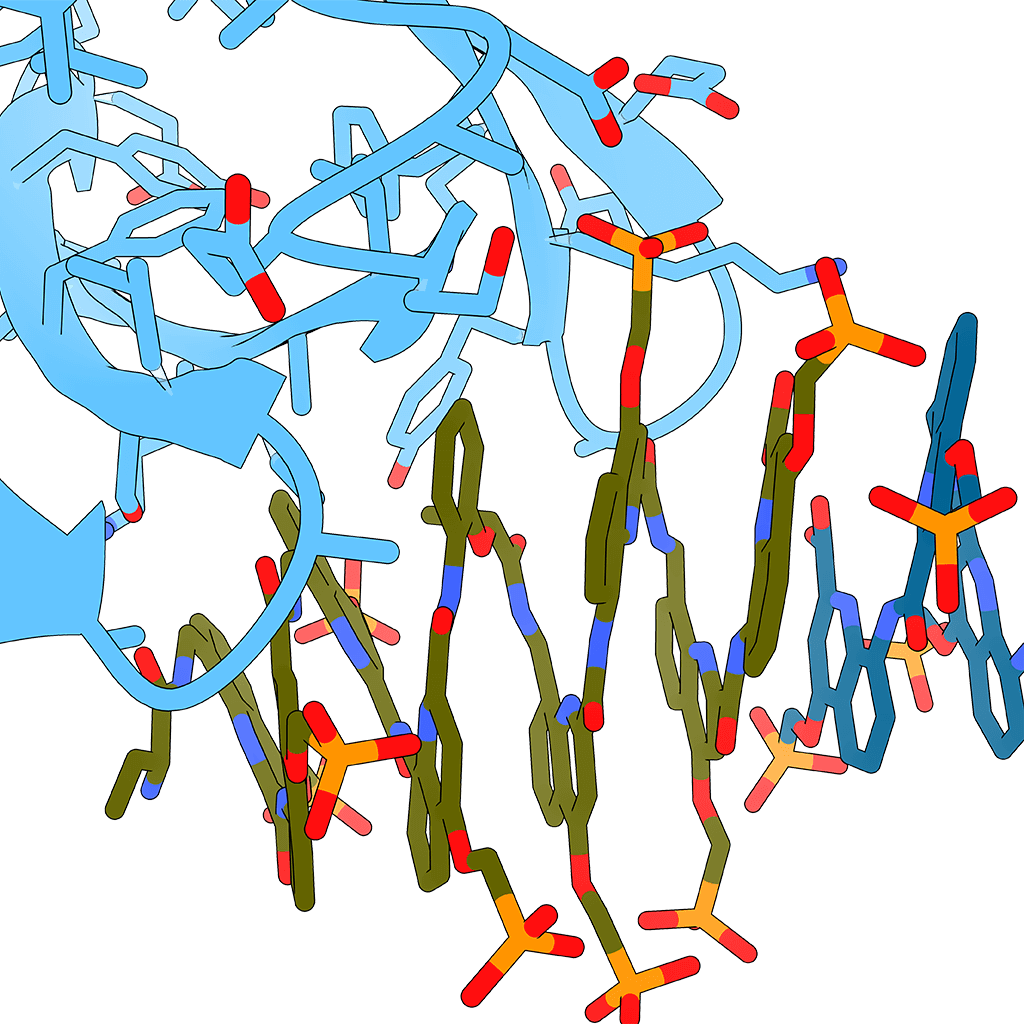
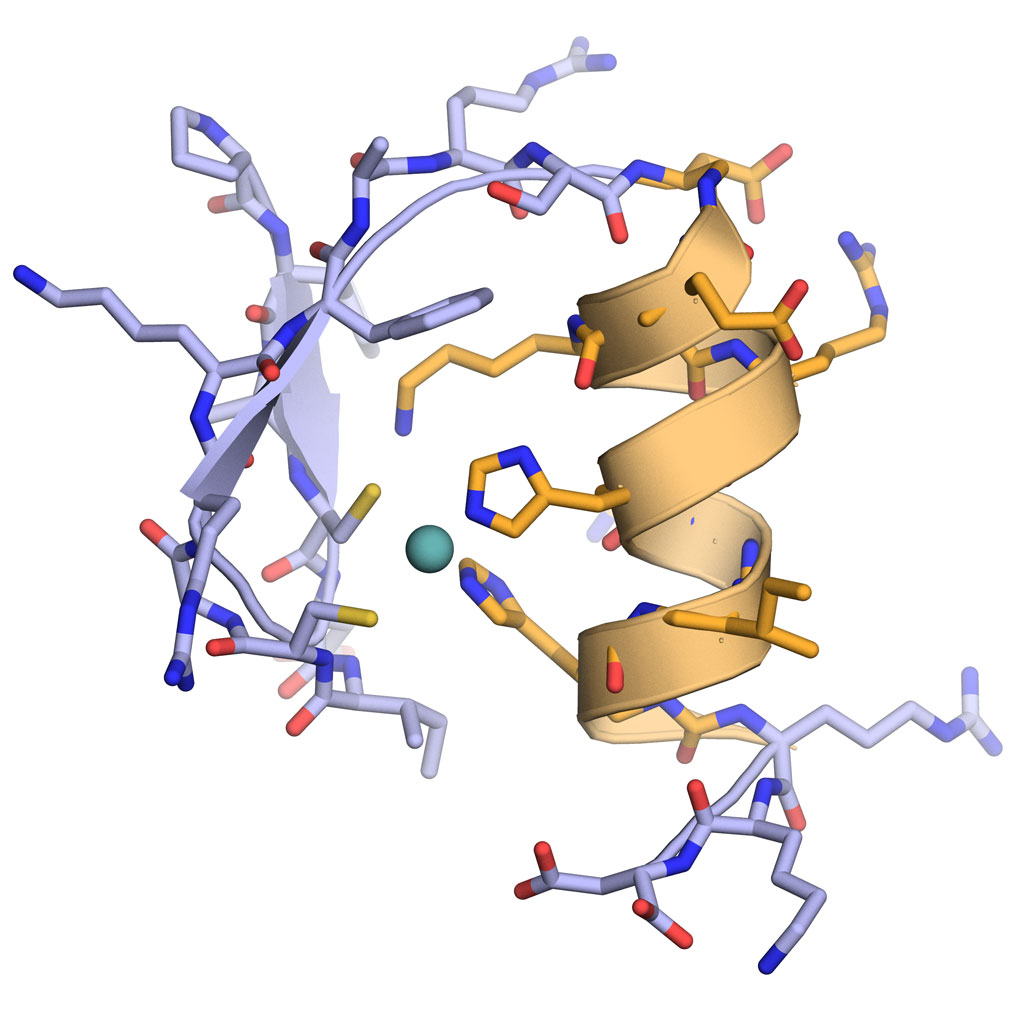
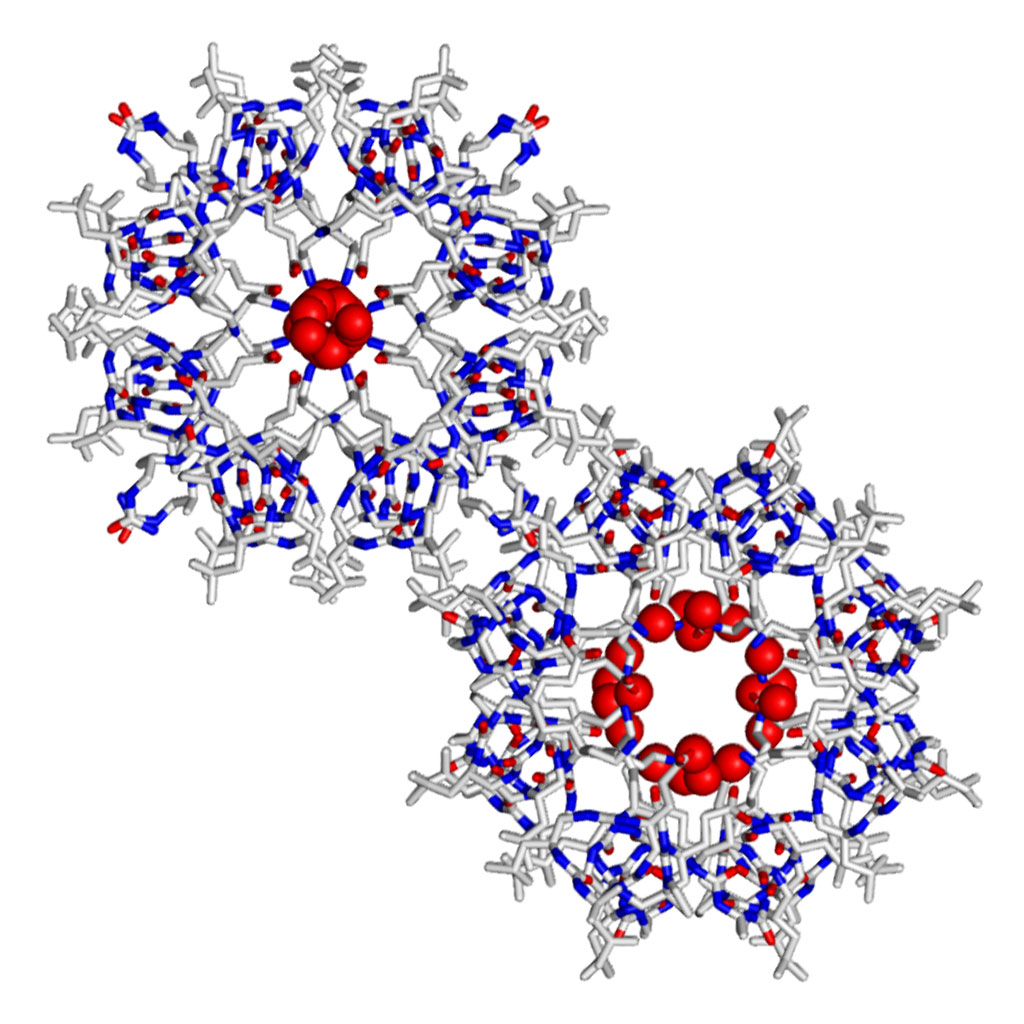
Like peptides and oligonucleotides, when a foldamer folds into a discrete structure, it can place functionalities that are non-adjacent within the sequence into close proximity in three-dimensional space.¹ The possibilities that this can bring about are vast – the most obvious being any molecular recognition that requires a precise arrangement of functionality. Examples would include peptidomimetics – the disruption of pathogenic protein-protein interactions – where a foldamer can mimic one of the partners², through to catalysis³, where functional group arrangement can orchestrate reaction processes, and of course host-guest chemistries, where the precise cavities and functionality within a foldamer make it the perfect host to small molecule with a defined stereochemistry. The greater diversity of structures there are, and the more chemical space explored, the more potential interactions available to us.
But the potential applications go beyond this. They have been shown to act as molecular information transfer systems, and have been used in the fabrication of electroluminescent devices, conformational switching, and systems that are capable of light-responsive hydrogen bonding.
- (a) Gellman, S. H. Acc. Chem. Res., 1998, 31, 173.
(b) Hill, D. J.; Mio, M. J.; Prince, R. B.; Hughes, T. S.; Moore, J. S. Chem. Rev. 2001, 101, 3893.
(c) Yashima, E.; Ousaka, N.; Taura, D.; Shimomura, K.; Ikai, T.; Maeda, K. Chem. Rev. 2016, 116, 13752.
(d) Goodman, C. M.; Choi, S.; Shandler, S.; DeGrado, W. F. Nat. Chem. Biol. 2007, 3, 252.
(e) Huc, I. Eur. J. Org. Chem. 2004, 17.
(f) Rao, S. R.; Schettler, S. L.; Horne, W. S. ChemPlusChem 2021, 86, 137. - Sang, P.; Shi, Y.; Huang, B.; Xue, S. Y.; Odom, T.; Cai, J. F. Acc. Chem. Res. 2020, 53, 2425.
- (a) Legrand, B.; Aguesseau-Kondrotas, J.; Simon, M.; Maillard, L. Catalysts 2020, 10, 700.
(b) Girvin, Z. C.; Gellman, S. H. J. Am. Chem. Soc. 2020, 142, 17211.
Join the UK Foldamer Network
To engage with all the exciting opportunities and events this network has to offer, please register now. It’s quick and easy.